COVID-19 Vaccine Approved for Children Ages 5-11
November 5, 2021
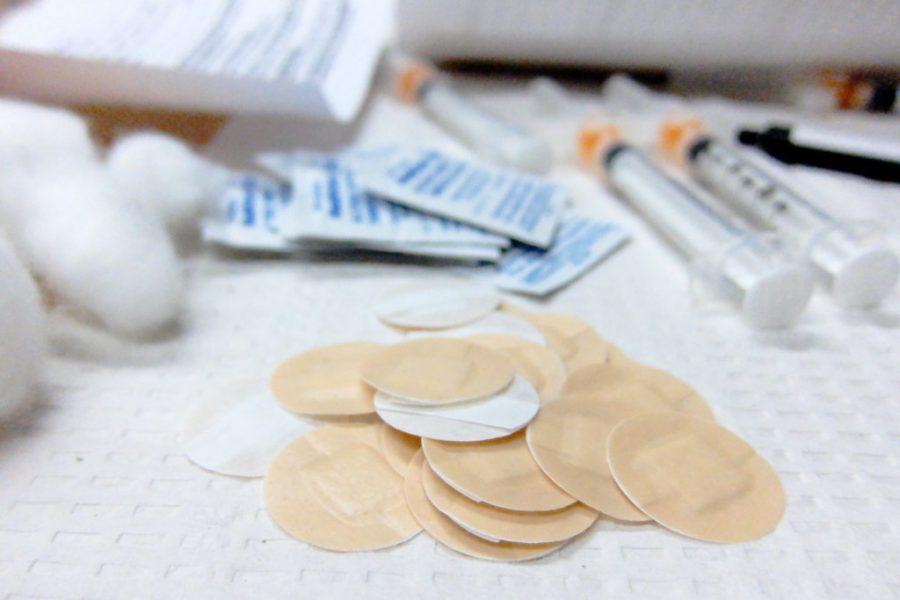
Tuesday, Nov. 2, federal health officials gave final approval to using the Pfizer-BioNTech vaccine for ages 5-11.
The Food and Drug Administration (FDA) determined that the Pfizer-BioNTech Vaccine has met the safety and efficiency standards for authorization in children ages 5-11. Advanced Practice Providers (APP) released a policy statement recommending that all eligible children get vaccinated.
The final approval came four days after the FDA granted emergency use of the vaccine for children ages five and up. Director Rochelle P. Walensky of Centers for Disease Control and Prevention (CDC), signed off on the use of COVID-19 vaccine in children ages 5-11 following a 14-0 vote in favor by the agency’s Advisory Committee on Immunization Practices (ACIP).
The safety of the COVID-19 vaccines continues to be monitored.